MIT-Chemiker entdecken, warum die photosynthetische Lichtgewinnung so effektiv ist
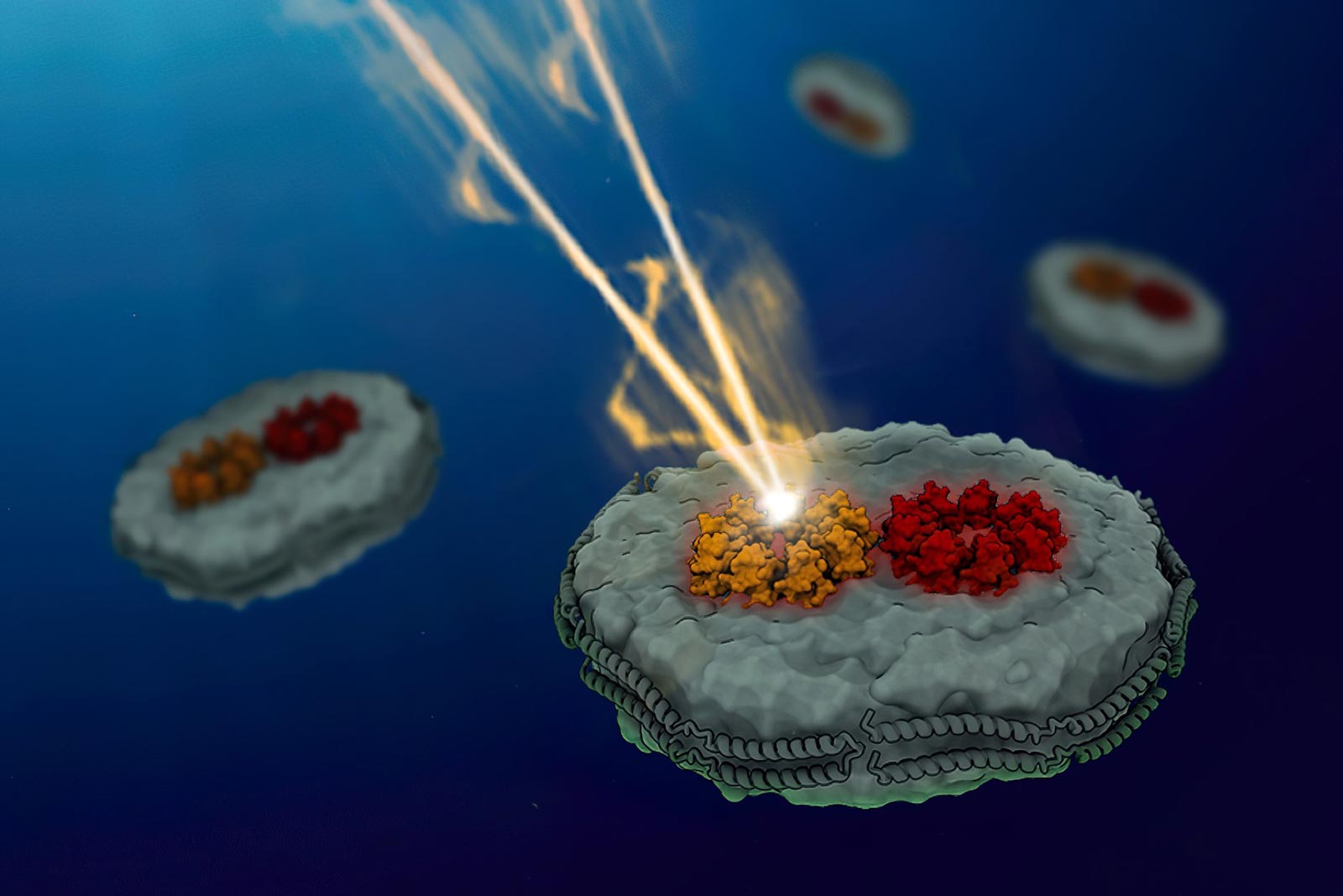

Zum ersten Mal haben MIT-Chemiker den Energietransfer zwischen photosynthetischen Proteinen, die Licht sammeln, gemessen und dabei entdeckt, dass die ungeordnete Anordnung lichtsammelnder Proteine die Effizienz des Energietransfers steigert. Bildnachweis: Mit freundlicher Genehmigung der Forscher
Die ungeordnete Anordnung von Proteinen in Lichtsammelkomplexen ist der Schlüssel zu ihrer maximalen Effizienz.
When photosynthetic cells absorb light from the sun, packets of energy called photons leap between a series of light-harvesting proteins until they reach the photosynthetic reaction center. There, cells convert the energy into electrons, which eventually power the production of sugar molecules.
This transfer of energy through the light-harvesting complex occurs with extremely high efficiency: Nearly every photon of light absorbed generates an electron, a phenomenon known as near-unity quantum efficiency.
A new study from MIT chemists offers a potential explanation for how proteins of the light-harvesting complex, also called the antenna, achieve that high efficiency. For the first time, the researchers were able to measure the energy transfer between light-harvesting proteins, allowing them to discover that the disorganized arrangement of these proteins boosts the efficiency of the energy transduction.
“In order for that antenna to work, you need long-distance energy transduction. Our key finding is that the disordered organization of the light-harvesting proteins enhances the efficiency of that long-distance energy transduction,” says Gabriela Schlau-Cohen, an associate professor of chemistry at MIT and the senior author of the new study.
MIT postdocs Dihao Wang and Dvir Harris and former MIT graduate student Olivia Fiebig PhD ’22 are the lead authors of the paper, which appears this week in the Proceedings of the National Academy of Sciences. Jianshu Cao, an MIT professor of chemistry, is also an author of the paper.
Energy capture
For this study, the MIT team focused on purple bacteria, which are often found in oxygen-poor aquatic environments and are commonly used as a model for studies of photosynthetic light-harvesting.
Within these cells, captured photons travel through light-harvesting complexes consisting of proteins and light-absorbing pigments such as chlorophyll. Using ultrafast spectroscopy, a technique that uses extremely short laser pulses to study events that happen on timescales of femtoseconds to nanoseconds, scientists have been able to study how energy moves within a single one of these proteins. However, studying how energy travels between these proteins has proven much more challenging because it requires positioning multiple proteins in a controlled way.
To create an experimental setup where they could measure how energy travels between two proteins, the MIT team designed synthetic nanoscale membranes with a composition similar to those of naturally occurring cell membranes. By controlling the size of these membranes, known as nanodiscs, they were able to control the distance between two proteins embedded within the discs.
For this study, the researchers embedded two versions of the primary light-harvesting protein found in purple bacteria, known as LH2 and LH3, into their nanodiscs. LH2 is the protein that is present during normal light conditions, and LH3 is a variant that is usually expressed only during low light conditions.
Using the cryo-electron microscope at the MIT.nano facility, the researchers could image their membrane-embedded proteins and show that they were positioned at distances similar to those seen in the native membrane. They were also able to measure the distances between the light-harvesting proteins, which were on the scale of 2.5 to 3 nanometers.
Disordered is better
Because LH2 and LH3 absorb slightly different wavelengths of light, it is possible to use ultrafast spectroscopy to observe the energy transfer between them. For proteins spaced closely together, the researchers found that it takes about 6 picoseconds for a photon of energy to travel between them. For proteins farther apart, the transfer takes up to 15 picoseconds.
Faster travel translates to more efficient energy transfer, because the longer the journey takes, the more energy is lost during the transfer.
“When a photon gets absorbed, you only have so long before that energy gets lost through unwanted processes such as nonradiative decay, so the faster it can get converted, the more efficient it will be,” Schlau-Cohen says.
The researchers also found that proteins arranged in a lattice structure showed less efficient energy transfer than proteins that were arranged in randomly organized structures, as they usually are in living cells.
“Ordered organization is actually less efficient than the disordered organization of biology, which we think is really interesting because biology tends to be disordered. This finding tells us that that may not just be an inevitable downside of biology, but organisms may have evolved to take advantage of it,” Schlau-Cohen says.
Now that they have established the ability to measure inter-protein energy transfer, the researchers plan to explore energy transfer between other proteins, such as the transfer between proteins of the antenna to proteins of the reaction center. They also plan to study energy transfer between antenna proteins found in organisms other than purple bacteria, such as green plants.
Reference: “Elucidating interprotein energy transfer dynamics within the antenna network from purple bacteria” by Dihao Wang, Olivia C. Fiebig, Dvir Harris, Hila Toporik, Yi Ji, Chern Chuang, Muath Nairat, Ashley L. Tong, John I. Ogren, Stephanie M. Hart, Jianshu Cao, James N. Sturgis, Yuval Mazor and Gabriela S. Schlau-Cohen, 3 July 2023, Proceedings of the National Academy of Sciences.
DOI: 10.1073/pnas.2220477120
The research was funded primarily by the U.S. Department of Energy.

„Musikfan. Sehr bescheidener Entdecker. Analytiker. Reisefreak. Extremer Fernsehlehrer. Gamer.“





